
The Blood-brain barrier, the gatekeeper of the brain
by YING CHIEH WU
The brain is the most important organ in our body. Don’t get me wrong, I also admire the contribution from other organs, like the heart. After all, people are not ‘alive’ if the heart is not beating. However, the brain is crucial to our daily life. It controls the movement of our body, the secretion of hormones, and the processing of input information such as vision and auditory. Our body cannot function properly without the brain. Moreover, the brain is where consciousness comes from. People may fall into a coma if their brain gets infected or is injured, resulting in the entire body system shutdown.
You may panic now and want to ask, ‘what should I do to protect my brain?’. Do not worry. Your body keeps something up in its sleeve. A specific vascular structure termed the blood-brain barrier (BBB) is formed in the central nervous system (CNS) to protect our brain. The BBB comprises several cell types such as endothelial cells (ECs), pericytes, and astrocytes. ECs limit things that pass through by forming the tight junction proteins (TJPs) between cells. Imagine TJPs as hands of ECs: the holding hands between ECs establish a wall to prevent things getting into the brain, including pathogens and toxins. Pericytes and astrocytes are supporting cells in the vasculature. They cover the ECs layer and regulate the function of ECs via physical contact or cytokines (small proteins produced by cells and that can control cell function, immune system, and other activities in the body) production. In other words, astrocytes and pericytes are able to control the number of hands in ECs, and how tightly ECs hold their hands.
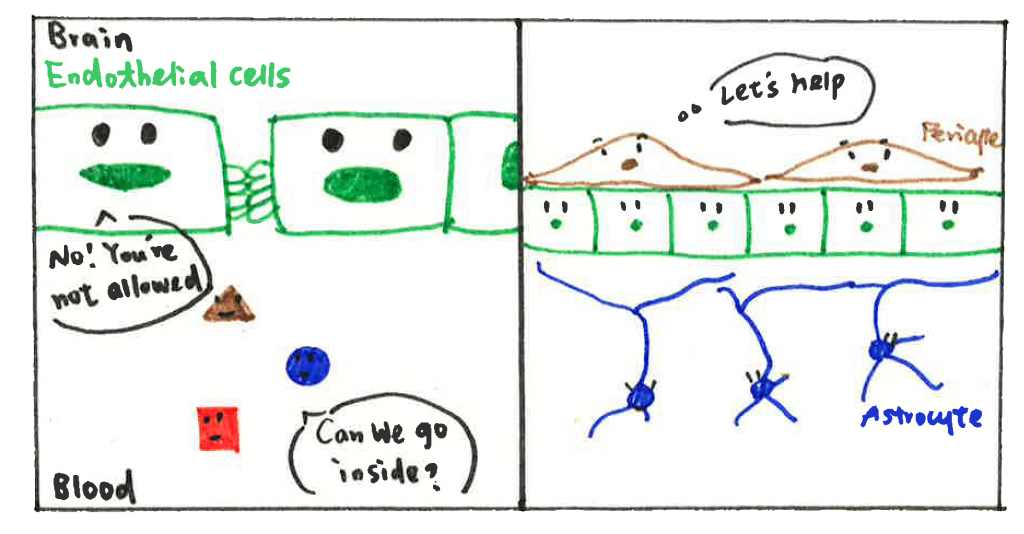
Actually, there is another partner in the BBB group, named perivascular macrophages (PVMs). I will introduce it later because PVMs have also been found and identified later in studies. Nevertheless, it does not mean it is unimportant. As an immune cell, PVMs are responsible for removing things that should not be present in the vasculature, including pathogens, cell debris (organic waste left over after a cell dies), and misfolding proteins (proteins do not form in correct conformation, which might be toxic to cells). PVMs work as a cleaner to keep the environment safe and clean, which is essential for other cell types to maintain BBB function.
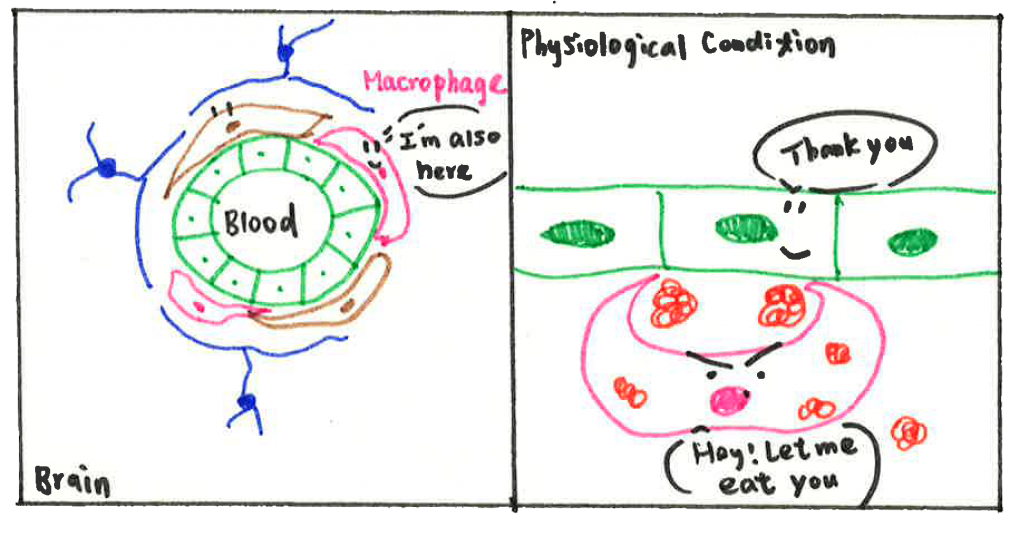
Unfortunately, the BBB is not invincible. In almost all kinds of brain disorders such as neurodegenerative disease, stroke, brain tumors, etc., the BBB is found disrupted and no longer able to protect our brain. The underlying mechanism of BBB breakdown is complicated; it could be ECs not holding their hands firmly anymore or astrocytes and pericytes not able to regulate the function of ECs. But here, I am going to provide some new perspectives from recent research. According to studies, scientists believe PVMs could be friends and foes to our brain depending on different circumstances. On the good side, PVMs help to remove depositions and maintain the vasculature environment, as we mentioned above. However, in brain disorders, PVMs are stimulated by aggregated proteins or due to signals from other cells and switch their status from ‘rest’ to ‘activated’. Once activated, PVMs can release inflammatory cytokines (a type of cytokine secreted from immune cells that promote inflammation) and induce oxidative stress that affects surrounding cells. Although cytokines secretion is one way the immune system fights against pathogens, it is also harmful to other cell types. Inflammatory cytokines are reported to decrease the expression level of TJPs (aka hands of ECs), resulting in increased permeability of the BBB. Hence, activated PVMs have also been considered as one potential mechanism for BBB dysfunction.
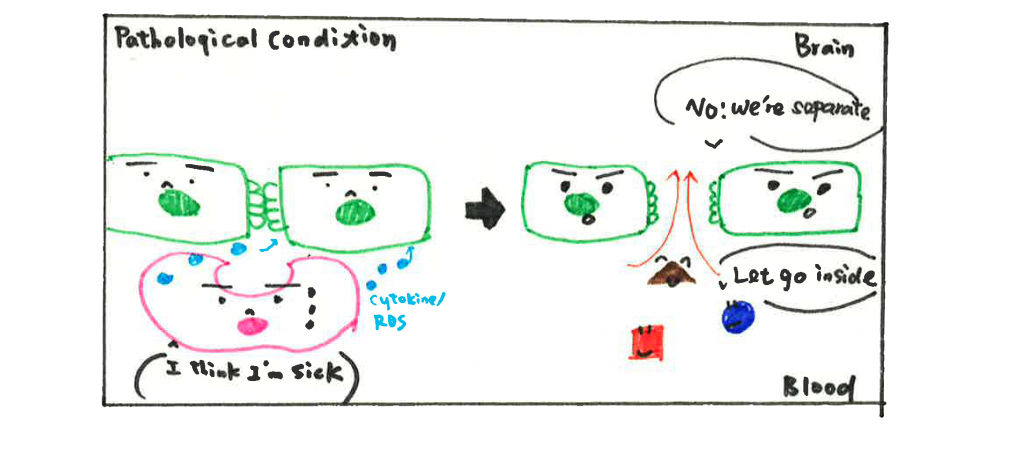
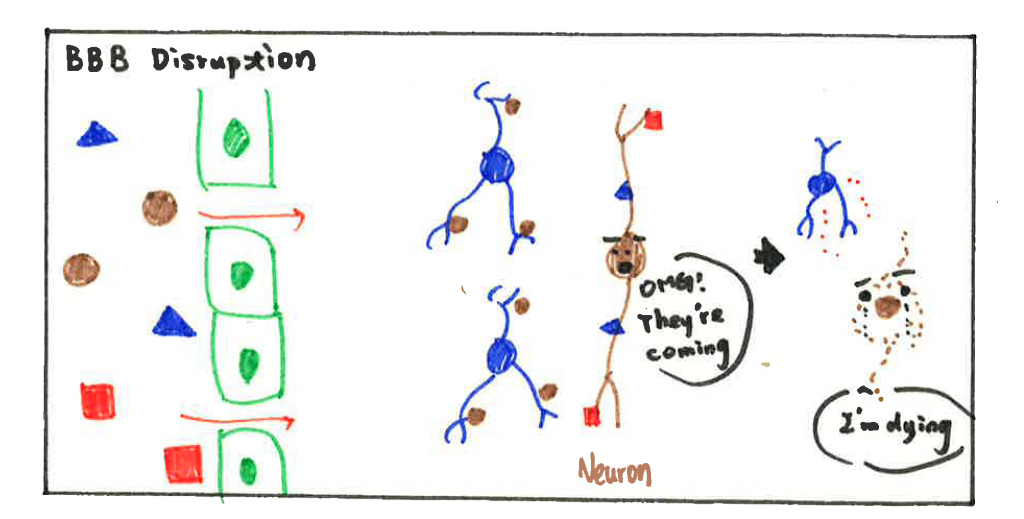
BBB disruption opens a door for toxins, pathogens, and immune cells from the peripheral (relative to CNS) circulation to get into the brain. The influx of immune cells and pathogens induces neuroinflammation (inflammatory response within the brain or spinal cord) and leads to neuron cell death, accelerating the progression of brain disorders. Taken together, PVMs seem to have a dual effect on BBB function, important in both physiological and pathological conditions.
Although evidence reveals that brain macrophages are close to ECs and play a role in maintaining BBB function, the underlying mechanism of their cross-talk is not well understood. Besides, it is not clear how they perform in the disease condition. Therefore, the ENTRAIN ITN program funded by the Horizon 2020 Framework Programme of the European Union was established for this purpose. We gathered experts from different countries and recruited 14 PhD students working on various aspects to figure out those critical questions. ESRs work on different disease models and use a different novel approach to investigate the interaction between endothelial cells and brain macrophages. We expect the results to let us know better about neuroinflammation and how brain macrophages regulate the BBB function and possibly find new promising targets for treating its future.
If you are interested in our program and want to learn more information, please visit our official website: https://itn-entrain.eu/
Although evidence reveals that brain macrophages are close to ECs and play a role in maintaining BBB function, the underlying mechanism of their cross-talk is not well understood. Besides, it is not clear how they perform in the disease condition. Therefore, the ENTRAIN ITN program funded by the Horizon 2020 Framework Programme of the European Union was established for this purpose. We gathered experts from different countries and recruited 14 PhD students working on various aspects to figure out those critical questions. ESRs work on different disease models and use a different novel approach to investigate the interaction between endothelial cells and brain macrophages. We expect the results to let us know better about neuroinflammation and how brain macrophages regulate the BBB function and possibly find new promising targets for treating its future.
If you are interested in our program and want to learn more information, please visit our official website: https://itn-entrain.eu/
references
- Giuseppe Faraco, Y. S., Diane Lane, Lidia Garcia-Bonilla, Haejoo Chang, Monica M Santisteban, Gianfranco Racchumi, Michelle Murphy, Nico Van Rooijen, Joseph Anrather, Costantino Iadecola (2016). “Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension.” J Clin Invest 126(12): 4674-4689.
- Laibaik Park, K. U., Lidia Garcia-Bonilla, Kenzo Koizumi, Michelle Murphy, Rose Pistik, Linda Younkin, Steven Younkin, Ping Zhou, George Carlson, Josef Anrather, Costantino Iadecola (2017). “Brain Perivascular Macrophages Initiate the Neurovascular Dysfunction of Alzheimer Aβ Peptides.” Circ Res 121(3): 258-269.
- Prat, R. D. a. A. (2015). “The Blood–Brain Barrier.” Cold Spring Harb Perspect Biol 7(1).
- Santa Mammana, P. F., Eugenio Cavalli, Maria Sofia Basile, Maria Cristina Petralia, Ferdinando Nicoletti, Placido Bramanti, and Emanuela Mazzon (2018). “The Role of Macrophages in Neuroinflammatory and Neurodegenerative Pathways of Alzheimer’s Disease, Amyotrophic Lateral Sclerosis, and Multiple Sclerosis: Pathogenetic Cellular Effectors and Potential Therapeutic Targets.” Int J Mol Sci 19(3).
- Takashi Koizumi, D. K., Toshiki Mizuno, Harry W. M. Steinbusch, and Sébastien Foulquier (2019). “Vessel-Associated Immune Cells in Cerebrovascular Diseases: From Perivascular Macrophages to Vessel-Associated Microglia.” Front Neurosci 13.
- Tobias Goldmann, M. J. C. J., Peter Wieghofer, Fabiola Prutek, Nora Hagemeyer, Kathrin Frenzel, Ori Staszewski, Katrin Kierdorf, Lukas Amann, Martin Krueger, Giuseppe Locatelli, Hannah Hochgarner, Robert Zeiser, Slava Epelman, Frederic Geissmann, Josef Priller, Fabio Rossi, Ingo Bechmann, Martin Kerschensteiner, Sten Linnarsson, Steffen Jung, and Marco Prinz (2016). “Origin, fate and dynamics of macrophages at CNS interfaces.” Nat Immunol 17(7): 797-805.