July 11, 2007, was not an ordinary day for Patient 3 as he was being wheeled into the operating room of Moorfields Eye Hospital in England. Patient 3, 18 years old, was crippled from birth with a disease known as retinal dystrophy. This inherited disease of the retina causes poor vision in childhood and complete loss of vision in early adulthood. There was no known effective treatment that could help improve his condition, until recently when his doctor suggested an experimental surgery. It was going to be a small surgery under general anesthesia. They said it wouldn’t take more than 1 or 2 hours. They were going to remove some of the jelly like liquid from his eye and inject a salty suspension under the retina. This suspension contained more than a billion particles of a genetically modified adeno-associated virus.
Retinal dystrophy is a genetic disease which leads to patients gradually losing their vision until one day darkness prevails. When we see an object, our eyes receive light signals. But our brain understands only the language of electrical signals. There is a need for translation of the signals and this is exactly what happens in our retina. The retina is a thin layer of tissue in the back of our eyeballs. Our retina has special cells called rods and cones which convert light signals into electrical signals which are then sent to our brain. Since these poor cells do a lot of work all day every day until we close our eyes and go back to sleep, they are always hungry for nutrients. There is a layer of cells in our retina just below these special translator cells whose job is to provide nutrients to these hard-working translators. But in some people, due to an error in their DNA code, these nutrient-providing cells fail to support the translator cells. Like workers ploughing a field under the burning sun for days without any food, these poor translator cells slowly starve to death, resulting in complete blindness in patients. One way to fix this problem is to somehow provide these nutrient-providing cells with the correct DNA code, so they can perform their job properly. But how do we deliver this correct instruction manual? We need a messenger, like how Hermes was to the gods of Olympus.
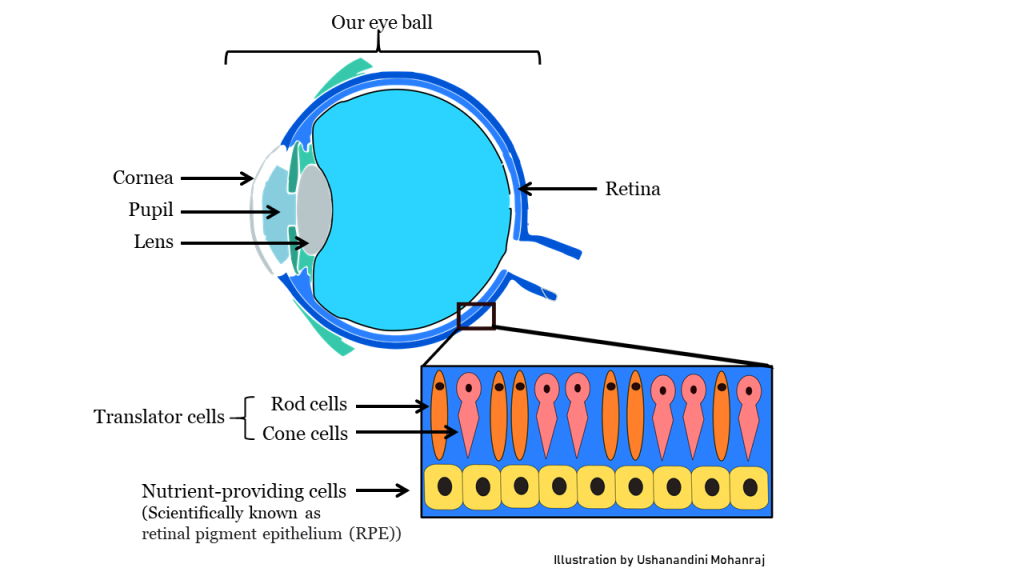
Our delicate yet wonderful eyes which helps us see the world we live in.
In our mortal world, we have viruses who are the perfect carriers of DNA messages. Viruses store DNA codes inside them, like an envelope with a letter. Once inside a cell in our body, our cell rips open the envelope, reads the letter and does what the letter says. But we cannot inject people with just any virus because many viruses make us sick. Scientists have two options to make this viral DNA delivery possible, they can either remove the dangerous parts of a virus that cause the disease and use the now safe virus for delivery. Or find viruses that, even though they infect us, do not cause any harm.
Viruses were first seen in an electron microscope in 1930. Thirty-five years later, a researcher at the University of Pittsburgh was performing a similar experiment for another virus called simian adenovirus type 15, in short, SV 15. One day, while studying the black and white image freshly extracted from an electron microscope, something was not making sense. The image was supposed to look like a polka dot print pattern, where the equally sized grey dots were the virus SV 15 on a black background. But this image was different, there were some smaller sized dots dispersed in between the bigger dots like a manufacturing defect. On repeating the experiment, with a higher magnification, now visualized more clearly, these hexagonal shaped particles turned out to be a novel virus called adeno-associated virus or AAV. Soon after, AAV drew the interest of many fellow curious minds from around the world, who embarked on a laborious journey to understand the biology of AAV, one of the smallest discovered virus back then. It was later understood that this virus did not cause any disease in humans but still infected almost any cell in our body, be it the cells of the liver, lungs, brain or eyes. This particular feature made AAV, the ideal delivery candidates. This eventually led to the first clinical trial injection of the messenger AAV carrying the correct DNA code, into a patient suffering from a deadly incurable disease in November 1995. Since then AAV based gene delivery method began to gain some momentum, giving hope to many. However, the popularity of this method did not last long enough.
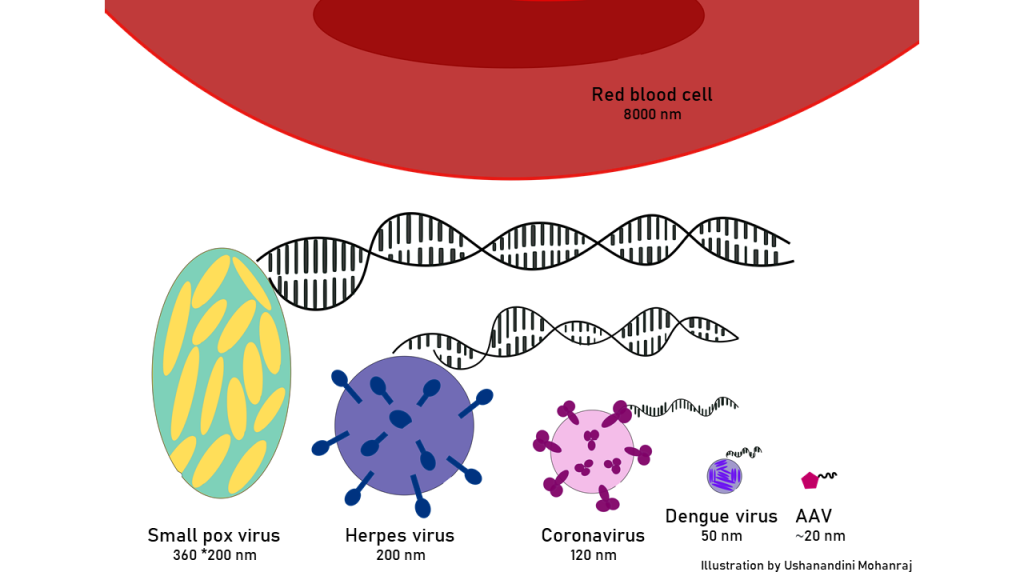
Fun fact: Viruses come in many different shapes and sizes. Here is the size comparison of some of the viruses compared to our red blood cell. AAV is one of the tiniest human infecting viruses.
On a fateful day in September 1999 in Philadelphia, viral therapy took the life of a 17-year-old Jesse Gelsinger. Jesse was suffering from a genetic disease of the liver and volunteered for adenovirus-based gene therapy. Adenoviruses which are different from AAV, were first approved for use in clinical trials in 1990. Being the 18th person to receive this modified virus in a clinical trial, he showed a very adverse reaction which quickly led to kidney, liver, and lung failure. Four days after receiving the viral shot, Jesse was declared brain dead and taken off life support. His case was widely publicized, and the trial researchers criticized for not being more cautious. Unfortunately, after this incident, a blanket of fear fell over the potential of gene therapy not just for adenovirus, but also for other viruses.
It was in this atmosphere of fear that Patient 3, back at Moorfields Eye Hospital in England, entered the operating room. Patient 3 was feeling nervous. The words from a disclosure contract that he had signed before the surgery loomed in his mind- “If taking part in this research project harms you, there are no special compensation arrangements.” He was aware that “harm” was a possibility. But on the other hand, he also wondered how much better his life would become if his eyesight improved. The doctors had explained to him that a lot of research has been done with AAV, it was known to be safe and that he would be monitored well after the surgery. Soon his mind wandered, and he couldn’t think clearly. The anesthesia started to take effect, he felt out of control but also calm, drifting into a state of nothingness. He soon fell asleep.
Six months later, patient 3 was waiting for his regular eye check-up at the clinic. The doctors wanted to make sure that everything was okay with his eyes since his surgery in July. Though the surgery did not make him completely recover from his condition, due to his age and disease progression, he was happy that it did improve his eyesight significantly. In these six months since the surgery, he was able to see better in low light and also discern the shapes and details of objects more clearly. He couldn’t have asked for a better Christmas present.
Ten years later, in december 2017, the US Food and Drug Administration (FDA) finally approved the first AAV based gene therapy for retinal dystrophy for clinical use. In 2019, FDA approved AAV based gene therapy for another disease called spinal muscular atrophy where patients experience muscle weakness and loss of muscle control. Many patients have been successfully treated ever since. The journey of AAV since its discovery in 1965 up until now was a very long one. It was only possible due to curiosity-driven research, a global collaborative effort, many success stories and some heart-breaking pitfalls.
References
1) Bainbridge, J. W., Smith, A. J., Barker, S. S., Robbie, S., Henderson, R., Balaggan, K., … & Ali, R. R. (2008). Effect of gene therapy on visual function in Leber’s congenital amaurosis. New England Journal of Medicine, 358(21), 2231-2239.
2) Atchison, R. W., Casto, B. C., & Hammon, W. M. (1965). Adenovirus-associated defective virus particles. Science, 149(3685), 754-755.
3) Wang, D., Tai, P. W., & Gao, G. (2019). Adeno-associated virus vector as a platform for gene therapy delivery. Nature reviews Drug discovery, 18(5), 358-378.
4) Nash, B. M., Wright, D. C., Grigg, J. R., Bennetts, B., & Jamieson, R. V. (2015). Retinal dystrophies, genomic applications in diagnosis and prospects for therapy. Translational pediatrics, 4(2), 139.
5) The Death of Jesse Gelsinger, 20 Years Later [WWW Document] (2019). URL https://www.sciencehistory.org/distillations/the-death-of-jesse-gelsinger-20-years-later